Gas chromatography is a process used to separate chemical
compounds for analysis. The analytical chemistry process is used with gases
that won't decompose when vaporized. Gas chromatography are used in a wide
range of industries -- everything from forensic science to medical marijuana.
While the procedure is highly useful, there are risks when working with
nitrogen gas. Learn how gas chromatography works, the role nitrogen plays, and
how an oxygen sensor improves safety.
How
Gas Chromatography Work
In chromatography, one gas moves over the sample substance. The
moving gas is known as the mobile phase, and it's usually an inert gas, such as
nitrogen or helium. As the mobile phase passes over the substance, it separates
out into its component parts. Since accuracy is key, it's vital that the moving
gas not react with the substance being analyzed. For this reason, inert gases
are recommended for gas chromatography.
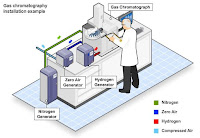
Gas chromatography takes place within a special machine, known
as a gas chromatograph machine. The substance being studied is injected
into the chromatograph with a syringe, then the material is heated to the vapor
stage. The carrier gas -- e.g. nitrogen -- is then added to the chromatograph
to push the sample up the central column. As the substance being analyzed
passes up the column, it's absorbed by the carrier and then separated into its
distinct components. The components emerge from the column and pass through a
detector, where they are identified and noted on a chart.
When the process is complete, every part of the mixture is
identified. At this point, for instance, a forensic scientist will have
the raw data needed to analyze evidence found at the crime scene. While
television shows may portray the process as instant, it's often time-consuming.
Within the medical marijuana industry, scientists are using gas
chromatography to test for pesticide residue in cannabis. While the medical
marijuana industry is still young, and pesticide levels are not heavily
regulated, industry leaders expect this to change as the marijuana industry
grows. Thus, the use of gas chromatography to check for pesticides will grow
too.
Whenever gases is used in the chromatography
process, there's a potential for gas leaks, whether from the supply lines,
storage tanks, or from the chromatograph itself. Nitrogen gas displaces oxygen.
If nitrogen were to leak, air levels would become deficient of oxygen and
employees could suffer health problems.
Since nitrogen gas has no color or odor, there is no way for lab
staff to tell that the gas has leaked. The best way to safeguard the lab is
with an oxygen monitor.
How
an Oxygen Deficiency Monitor Protects Employees
Risks of breathing oxygen deficient air include dizziness,
fatigue, unconsciousness, and death via asphyxiation. All it takes is a couple
breaths of air to experience adverse health effects.
Since there is no way to tell whether a leak has occurred, it's
necessary to use an oxygen sensor to track oxygen levels at all times. The
oxygen monitor or sensor measures oxygen and only reacts when levels fall below
a predefined threshold. Oxygen sensors from PureAire have alarms for
oxygen levels of 18 percent and 19.5 percent, for instance.
The oxygen deficiency monitor includes a flashing light and loud
alarm, so that staff and passerby receive prompt notification of the leak. When
the alarm goes off, employees can vacate the premises and contact emergency
personnel.
Given the serious risks posed by a nitrogen gas leak, it's
important to use oxygen deficiency monitors anywhere inert gases are stored or
used.
PureAire is an industry leader when it comes to oxygen
monitors. O2 monitors from PureAire are designed for
long-lasting and maintenance-free use. They feature a zirconium sensor, which
lasts for 10-plus years without calibration. PureAire's monitors can
handle temperature changes, barometric shifts, and even freezing
temperatures. Learn more about PureAire's monitors and how they
promote safety at

No comments:
Post a Comment